MATTER
DEFINITION-The things that have mass and occupy space are called matter.
Classification of matter -
Some Old philosophers have been classified matter into five basic elements . These are as follows....
1-Air
2-Earth
3-Fire
4-Sky
5-Water
In modern era, Matter has classified into three states solid ,liquid and gas.
Physical nature of matter-
Particles are smalller in size
a-Matter is made up of tiny particles, this can be understood by this activity that when we dissolve a pinch of sugar or salt in small amount of water then we find that sugar or salt molecules are disappeared soon.
Salt
Water
Sugar Solution
fig-1
Salt Solution
fig-2
An Activity showing,matter is made
up of particles
b- If we take a pinch of Potassium permagnate and dissolve it in 100 ml water then water becomes red in colour and take 10 ml from it then add 90 ml. of water further take 10 ml. from this solution and add 90 ml of water repeat this process five times we find the colour of the solution becomes light successively. This shows that particles are v v small in size.

Activity showing particles are
smaller in size
Characteristics of matter
Particles of matter have space b/w them
when we dissolve the Potassium permagnate in water the colour of water becomes red because Potassium permagnate (red colour) is in solid form or in crystalline form.On the otherhand water is in liquid form, the density of water is lower than the density of solid so there is more space between the particles of water than the Particles of Potassium per magnate this is why the paricles of KMnO୳ comes between the particle of water and a Solition is formed.
Particles attract each other
Particles attract each other. The
Different matters have different type of bonds by which the particles of matter are joined to each other.
Tne particles of solid have strong
bonds amongs themslves while the bonds of liqid are not so strong. In gases the bonds are weak. Bonds in solids are ionic or covalent bonds while in liquid covalent, polor and hydrogen bonds are found. On the other hand bonds in gases are covalent and wanderwal forces.
The Particles of matter are in continuous motion.
This can be shown by the follwing activity....
1-Activity related to incence stick
when incence stick is kept in the corner of a room and we sit at another corner then we feel a pleasent smell sitting at a distance also.
2-Activity related to red ink and honey
when we take two beaker , both are filled with water and in the first beaker put a drop of red ink. In the same way ,in the second beaker put honey drop and remain the beaker undisturbed.
After adding the ink we observe that the ink is spreading v fast while in the second beaker Honey takes v much time to be spread out. This shows that particles of matter are continuously moving and speed of particles of liquid (ink) is more than the particles of honey.
3-Activity related to sache of scent
When we open the sache or small boltle of itra or scent in the room what happens , smell spreads all around the room.
Effect of temperature on solubility of matter
1- Take hot water and cold water.
2- Take Potassium Permagnate
on dissolving Potassoum permagnate in both. Observe What happens
Observation-In hot water Potassium
Parmagnate dissolve soon while in cold water it takes time to be dissoved. So it is clear that on increasing temperature solubility or mixing ability of matter increases.
Note-:When max. amount of solute dissolve in the solvent at particular temperature , it is known as solubility.
In activity b -we took 10 ml from already prepared solution and add 90 ml. water. We do it many times suppose five times but in thisprocess the amount of solvent(matter that is taken in more amount than other and here this is water) increasing continuously while the amount of the solute ( substance that is taken in lesser amount and here this is Potassium permagnate) is decreasing in comparison to water this is why less nos. of KMnO୳ molecules will be adjust between lot of molecules of water. So each time on taking 10 ml. Solution and adding 90 ml. water will give us a fad coloued solution.Kinetic Energy-
In gas The kinetic energy is highest while Potential energy is lowest. On increasing temperature, Velocity of particles increases, Resultantly kinetic energy also increases.....
v∝√T
pic (a )shows low speet because of low temp while (b ) pic show high speed of molecules due to high temp.(temp=temperature) 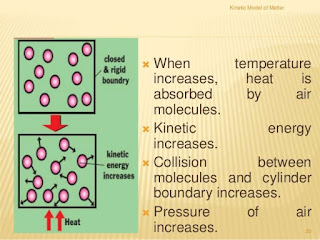

in above figure
P∝T
in above figure At constant temp, volume decrease the pressure increases. T =constant
P∝1/V
In liquids- The Kinetic energy of the particle of liquids is lower than gas but higher than solid.
In solid -Ptential energy is highest but kinetic energy is lowest.
Note- The Particles of solid can not move freely because they are tightly bonded so they show vibratory motion. The kinetic energy in solids is due to their vibratory motion only.
States of matter-
There are generally three states of matter observed by the scientist these are as follows-
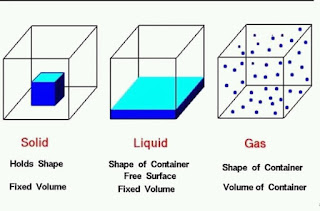
Solid-
If it is a metal , then bonds formed so among the atoms will be called matellic bonds and these bonds forms a lustre or net of bonds this is called metallic lustre. Besides this there may be molecule of compound and these molecules are joined by the chemical bonds. These bonds are as follows
1- covelent bond
2- ionic bond
3- co-ordinate bond
Covalent bond- These are formed by the sharing of electrons
Ionic bond- These bonds are formed by transferring the electrons. In this transferring, positive and negative ions are formed. Molecule of Ionic solids are held by the the positively and negatively charged ions
Co-ordinate bond-In this type of bond electrons are kept in such a way that pair of electrons (given by donor ) is shared by both donor and receiver.
A liquid is nearly incompressible fluid. It can flow and possesses viscosity (viscosity is a property of liquid by which it comes to rest during flow due to resistance among layers).
Diffusion rate in liquid is lower than gases but higher than Solid. Each liquid has its definite boiling point. Such as boiling point of water is 100°C. Above it water starts to convert into Vapour. This process is called vapourization.
Gas
There are differnt gases in the atmosphere.Gases are highly compressible as compared to Solids and liquids. Gases (in the cylinder for cooking called LPG and oxygen filled in cylinders for hospital patient) are highly compressed gas. CNG is used as fuels in the vehicles now a days
CNG =Compressed Natural Gas
LPG= Liquid Petroleum Gas
Due to high compressibility , large volumes of gas can be compressed into small cylinders so as to make transportation easily.
In the gaseous state,the particles move about randomly at high speed. Due to this random motion , the particles hit each other and also hit the walls of the container. The gas particles exert force per unit area on the walls of the container. Due to this force per unit area, a pressure gives rise.
Solid-The volume and shape of the solid is fixed. This is due to strong bonding among the particles.
Liquid-The volume of the liquid is fixed but not shape because bonds of particle of liquid are not so strong as in Solid. in which pot we pour the liquid it takes the shape to that.
-Neither Volume nor shape is fixed in gases. becausee the kinetic energy of molecules of gas is highest among Solid, liquid and gas.

Now a days the scientists have talked about the five states of matter:
1-Solid
2-Liquid
3-Gas
4-Plasma
5-Bose-Einstein Condensate
We have given the knowledge about three states of matter. We will give description of Plasma and Bose- Einstein Condensate...
Plasma-This state consists ofSuper energetic and Super excited Particles. Actually these particles are of ionised gas like neon and helium or some other gases. These gases are filled in the sign tubes or other fluorescent tubes. The ions of thes gases in the tubes get charged when electricity is flown in the tube. Due to charging up of ions ,Plasma glows. Sun and Stars glow due to presence of Plasma. The Plasma is created on the stars due to very high temperature.
Bose Einstein condensate-In 1920 indian Physicist Satyendra Nath Bose had made some calculations for fifth state of matter. On the bases of S N Bose calculations, Albert Einstein predicted a new state of matter. In 1955 Eric A. cornell Wolfgang ketterle and Carl E. Wieman of USA created this fifth state of matter and received the noble prize in physics for achieving "Bose-Einstein condensation." in 2001.
Lowest energy of Bose-Einstein Condensate.
Plasma contains highest energy.
Arrow shows increasing order of energy.


















Comments
Post a Comment