SYMBOLS OF CHEMICAL ELEMENTS
Symbol- Abbreviation of elements in the form of one or two letters is called Symbol, eg.Hydrogen=H, Helium=H
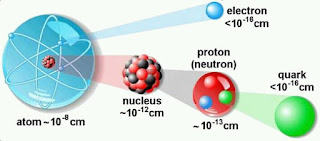
A diagramatic representation of Atom
Note-Atom is the the smallest particle of any Element. Radius of atom is ten the power minus eight cm. ,While Nucleus is the centre of the atom and its radius is 10 the power minus 12 cm.
History of Symbol-
In 1803, An English Chemist and Physicist, John Dalton gave the Symbols first to represent the atoms of the elements, besides this Dalton was considered as the father of modern atomic theory. He told that molecules and atoms may be represented in Symbolic form in place of writing their names but now a days Dalton's Symbols are not recognised. As we know the era is changed, In the same manner old Symbols (Symbols given by Dalton) are changed.
A Table of Symbol given by Dalton
Drawbacks of Dalton's sSymbols-
Dalton's Symbols are not used in the life of Chemistry due to following reasons...
a- The Symbols represented by Dalton are either in the form of only figure , only letter or both together. This is not so easy to remember those in comparison today's Symbols.
b-Dalton's Symbol can not be depicted in the form of table because these take more space, due to that there were a hochpoch in the table. The Dalton's table would not be learn able so easily as It is today (modern periodic table).
c-Dalton's Symbol method can not give rise the interest to learning as modern periodic table.
d- In Dalton's Symbol hydrogen is in the form of circle with dot in the middle. with this figure we can not attach atomic no. and atomic mass side wise.
e-We can not depict chemical equations with the help of Dalton's Symbol.
Definition of Symbol-Symbol is a short way of representing the elements. In other word we can say that the abbreviation of the chemical elements is called Symbol. for eg. Symbol of Hydrogen is H and symbol of Hydragyrum (Latin name of Mercury) is Hg.
A Figure of Mercury
Note-Density of Mercury is more than the density of water. if we take same volume to both mercury will be heavier than water. Remember Mercury is a metal in liquid state while Water is a liquid compound.
Forms of Symbol....
1- Monosybolic form
2-Diasymbolic form
Monosymbolic form....In this form of symbol there is only one letter by which any person can recognise the element what that is. eg Symbol of kalium (Latin name of potassium)
is K and for oxygen is O.
Symbol of Potassium
Diasymbolic form... ..In this type of form two letters are used as symbol. First letter of Symbol is taken as the first letter from the name of the element and the second letter will be just later letter or meddle letter of the name of element. Either the name of element is Latin name or other language name.
Note-First letter of Symbol should be as capital while second letter is small. eg. Sb for Stibium (Latin name of element) while its common known name is Antimony. This Symbol is taken from Latin name.
on the other hand Ne for neon .
A Picture representing Neon Atom
Advantages of Symbols-
1- With the help of symbols we can denote the Chemical formula of any compound like NaCl (Sodium Chloride)
HCl (Hydrogen Chloride).
2- By taking chemical formulae we can show the Chemical reactions.
NaOH + HCl ➡️ NaCl + ΗշO
Note-When Sodium hydroxide react with Hydrochloricacid, after reaction Sodium Chloride and water are formed.
3-Due to the applications of formulae we can write many equations in a very short space and time elapsed in writing the equations is v much less.
4-We can express the mass and atomic no. of an element in a convenient way as շHe༥ ( 2 left at bottom 4 right at top) or in the following manner...
5- A person can express all 118 elements as a table that is known as Periodic table in a less beleaguered space.
A Modern Periodic Table
Note-Modern Periodic table is the table in which all 118 Elememts are placed in Systematic and Proper way.
Besides above some other advantages of Symbols are also seen such as
Medical store or Chemical stores or the stokist of medicins and Chemicals.
Name of Elements are kept in the name of the place from where the elements are found or in the name of its discoverers.
Now we are going to make you study of 30 elements with atomic no., atomic mass, Symbol and their valancies.

A table for Atomic no. 1 to 30 Elements
Note-For the students it is very much important to take glance these 30 elements day thrice in a day like medicinal doses.
Besides above thirty elements some following symbols should also be remebered always because the compounds of theìse are also found well in nature .








fine description anout symbol
ReplyDeletethankyou
ReplyDeletethis helped me a lot thanks
ReplyDelete