Nucleic Acid
Nucleotides are the biomolecules which are composed of carbon ,hydrogen, oxygen,nitrogen and phosphorous. These are high energy compound. These are monomers and these form polymer of DNA or RNA by uniting to itself. DNA and RNA are genetic material or genetic molecules. These DNA and RNA molecules are biomolecules and found in all living things on this earth. These are basic building block. Lives can not exist without these molecules.
Composition of Nucleotide-
Nucleotide is composed of three molecules.
1-Nitrogenous base
2-Pentose Sugar
3-Inorganic Phosphate group
Inorganic Phosphate group
Composition of Nucleoside-
Nucleoside is composed of two molecules.
1-Nitrogenous base
2-Pentose sugar
so we can say....
Nucleoside=Nitrogenous base+sugarNucleotide =Nitrigenous base +sugar + Phosphate group or
Nucleotide=Nucleoside+Phosphate group
Deoxyribonucleotide=nitrogenous base+deoxyribose sugar + phosphate group.
Ribonucleotide=nitrogenouse base+ribose sugar+Phosphate group
1-Pyrimidine type
2-Purine type
Pyrimidine type-
These are hexagonal heterocyclic ring. These are composed of four elements nitrogen, carbon, hydrogen and oxygen. This include three bases Thymine, Cytosine and Uracil.
Purine and Pyrimidine notrogenous bases
Pyrimidine type nitrogenous base
Purine type-These are hexagonal heterocyclic ring with pentagonal ring. These are composed of hydrogen oxygen nitrogen and carbon. This catagory includes two types of bases adenine and Guanine.
Types of Nitrogenous Base
Nitrogenous bases are of five types-
Cytosine-C
Thymine-T
Uracil-U
Adenine-A
Guanine-G
These above nitrigenous baese have aeromatic and heterocycle rings. These are sparingly soluble in water. In De-oxyribonucleic acid (DNA) above bases besides uracil are used with deoxyribose sugar and Phosphate molecule. RNA does not contain thymine but has all four bases with ribose sugar and Phosphate molecule.
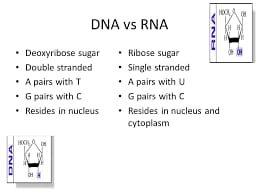
Difference b/w DNA and RNA..
DNA polymer contains thymine(not uracil)
and deoxyribose sugar while in RNA uracil (nitrogen base )and ribose sugar (not deoxyribose sugar)are found
Difference b/w riobose and deoxyribose sugar

both sugars are same in structure but difference is of oxygen atom. Deoxyribose sugar is short by one oxygen atom.
DNA is double stranded or we can say deoxyribonucleotides makes two polymers chain on both sides and When these polymers are jointed by nitrogenous bases face to face with the help of hydrogen bonds, deoxyribonucleic acid is formed.
DNA double stranded and RNA single stranded form (a)
RNA coiled and double stranded at some place (b)
Double Helix Model of DNA
First of all, in 1953 Francis Crick and James D. Watson gave Double Helix Model of DNA. They told that how the deoxyribonucleotide are joined to each other and make a polymer and how thesr polymer joined yo each other.
DNA double helix model (1953) given by Watson and Crick

DNA Model
- it contain two Polynucleotides molecules wound each other.
- The backbone of each consists of alternating deoxyribose and Phosphate group (means at one Poplynucleotide chain, Phosphate then sugar then Phosphate ..so on.
- One Phosphate is bounded to 5' carbon of one deoxyribose sugar and the same phosphate is bounded to 3'carbon of the next.
- The two strands are "antiparallel" that is 5' to 3' while other runs 3' to 5'.
- Purine or Pyrimidine attached to the sugar are directed to the axis of the helix.
- Both base pair are bounded by hydrogen bonds.
- Adenine joints to thymine by two hydrogen bonds while Cytosine and Guanine are joint to each other by three hydrogen bonds.
- Uracil is absent in DNA Structure or helix.
- Distance between two base pair of two polynucleotide is 3.4 °A and Diameter of helix is 20 °A (1°A=10¯10 m).
- This is the β helical Structure of DNA and its one complete helix contain 10 base pairs. so one complete helix length is 34 °A.
Properties of Nucleic acid-
1-These are biomolecules.
2-These DNA and RNA both are genetic material.
3-Generally DNA is found in the nucleus and RNA in the cytoplasm and Ribosome.
Beside this DNA is also found in cytoplasm such as in tbe bacterial cell.
4-DNAs are found in some organelles of the cells such as in the chloroplast and Mitochondria.
Note-Chloroplast and Mitochondrion (pl. Mitochondria) are cell organelles.
DNAs have a capacity of duplication (to make its own copy on DNA template).When DNA makes a copy of RNA on its strand then this process is called Transcription. But in some Virus RNA is the genetic
material and can make DNA on its template. This is called Reverse Transcription. such as in Retrovirus. When RNA or DNA is formed on the template of DNA. In this process an enzyme work this enzyme is called DNA Polymerase. If DNA is formed on RNA template (Reverse transcription), RNA Polymerase works. Polymerase joints the nucleotides to make Polymer of nucleotide or nucleic acid.
Central Dogma-Central dogma of molecular biology describes the two step process-Transcription and Translation, by which onformation in pgenes (small parts fl of DNA) flows into proteins.
DNA➡️RNA➡️PROTEIN
Importance of Nucleic Acid-
1-Nucleic acid carry the information from one generation to another generation.
2-Nucleic acid has the information in the form of chemical messeages that found in DNA then RNA (DNA transcript RNA) then Protein is formed.This Protein plays very important role in functional and structural deeds of the organisms.
3-DNA is responsible for the Primary and Secindary (Physical and Sexual ) Characteristics. On the other hand the work of RNA is to build Protein by uniting amino acids. Whatever be alternately RNA is also dependent on DNA.
4-DNA follows crossing over event during cell division, due to this new combinations are produced in the DNA. Resultantly the characteristics of organisms are changed.
5-Every person has its unique DNA, so DNA fingerprinting is the biotechnique by which a culprit may be caught easily. And any offspring and Parents may be recognised by this technology.
1-These are biomolecules.
2-These DNA and RNA both are genetic material.
3-Generally DNA is found in the nucleus and RNA in the cytoplasm and Ribosome.
Beside this DNA is also found in cytoplasm such as in tbe bacterial cell.
Note-Chloroplast and Mitochondrion (pl. Mitochondria) are cell organelles.
DNAs have a capacity of duplication (to make its own copy on DNA template).When DNA makes a copy of RNA on its strand then this process is called Transcription. But in some Virus RNA is the genetic
material and can make DNA on its template. This is called Reverse Transcription. such as in Retrovirus. When RNA or DNA is formed on the template of DNA. In this process an enzyme work this enzyme is called DNA Polymerase. If DNA is formed on RNA template (Reverse transcription), RNA Polymerase works. Polymerase joints the nucleotides to make Polymer of nucleotide or nucleic acid.
Central Dogma
Central Dogma-Central dogma of molecular biology describes the two step process-Transcription and Translation, by which onformation in pgenes (small parts fl of DNA) flows into proteins.
DNA➡️RNA➡️PROTEIN
Importance of Nucleic Acid-
1-Nucleic acid carry the information from one generation to another generation.
2-Nucleic acid has the information in the form of chemical messeages that found in DNA then RNA (DNA transcript RNA) then Protein is formed.This Protein plays very important role in functional and structural deeds of the organisms.
3-DNA is responsible for the Primary and Secindary (Physical and Sexual ) Characteristics. On the other hand the work of RNA is to build Protein by uniting amino acids. Whatever be alternately RNA is also dependent on DNA.
4-DNA follows crossing over event during cell division, due to this new combinations are produced in the DNA. Resultantly the characteristics of organisms are changed.
5-Every person has its unique DNA, so DNA fingerprinting is the biotechnique by which a culprit may be caught easily. And any offspring and Parents may be recognised by this technology.













Comments
Post a Comment